Please note that this “testing-the-waters” communication is available under rule § 227.206 with the following disclosures:
- no money or other consideration is being solicited, and if sent in response, will not be accepted;
- no offer to buy the securities can be accepted and no part of the purchase price can be received until the offering statement is filed and only through an intermediary's platform;
- a person's indication of interest involves no obligation or commitment of any kind.
Mission Statement
Tregeutix Inc. ("Tregeutix") is a biotech company whose mission is to create a future where autoimmune diseases and allergies can be safely and effectively managed through the strategic use of beneficial microbiota* species to guide the immune system.
To achieve this goal, Tregeutix is developing a proprietary platform to discover beneficial microbes that can support antigen-specific regulatory T cells (Tregs**) using a combination of computational and experimental methods. By leveraging beneficial microbes, Tregeutix aims to restore the balance of the immune system and alleviate symptoms of autoimmune diseases and allergies, which are intrinsically antigen-specific pathologies.
*Microbiota is the collection of microorganisms that live in and on our bodies, such as bacteria, fungi, and viruses. The microbiota influences many aspects of health, including immune system. Some microbiota species can induce or support Tregs.**Tregs are a type of white blood cell crucial for maintaining immune tolerance and preventing unwanted inflammation. They can suppress the activity of other immune cells implicated in autoimmune diseases and allergies.
#This figure contains elements adapted from the image by macrovector on Freepik.
Market Overview and Projections
Autoimmune diseases and allergies are among the most common and costly chronic conditions in the world, affecting millions of people, representing a considerable unmet medical need. By 2025, the global autoimmune disease therapeutics and allergy treatment markets are projected to reach $153 billion and $40 billion, respectively.
Clinical and Regulatory Status of Microbiome and Treg Therapies
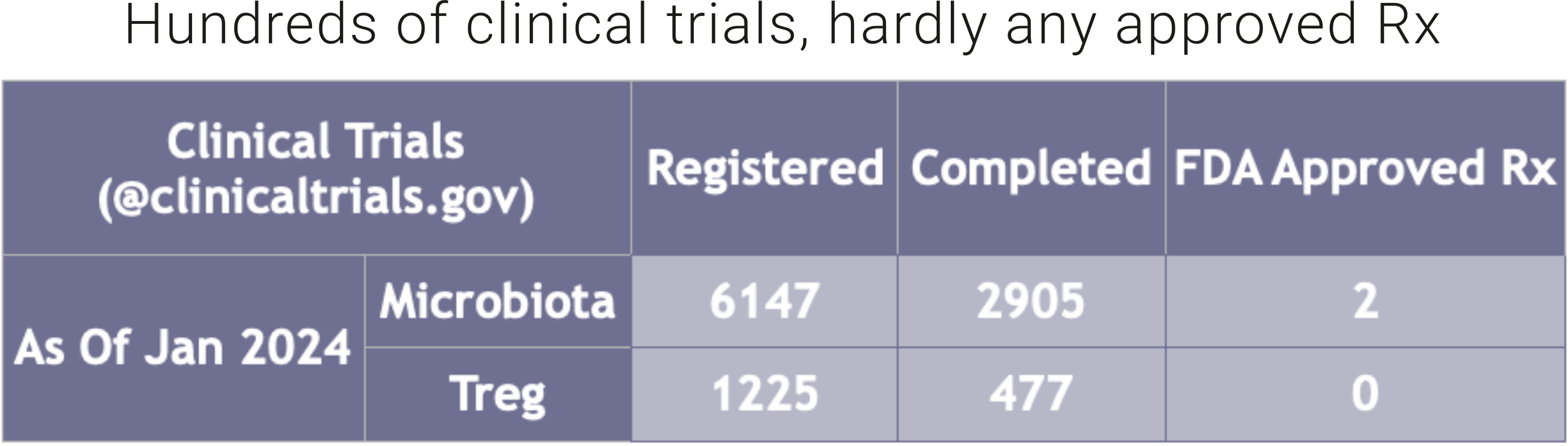
As of January 30, 2024, 6,147 clinical trials related to the microbiome (searched by microbiota OR microbiome OR probiotic) were registered on clinicaltrials.gov, of which 2,905 have been completed. Number of Microbiota product approved by the U.S. Food and Drug Administration (FDA): 2 (both products, Rebyota and Vowst, were approved for the prevention of recurrence of Clostridioides difficile infection (CDI)).
As of January 30, 2024, 1,225 clinical trials related to Tregs (searched by regulatory T cells OR Tregs) were registered on clinicaltrials.gov, of which 477 have been completed. Number of Treg products approved by the FDA: 0.
Challenges
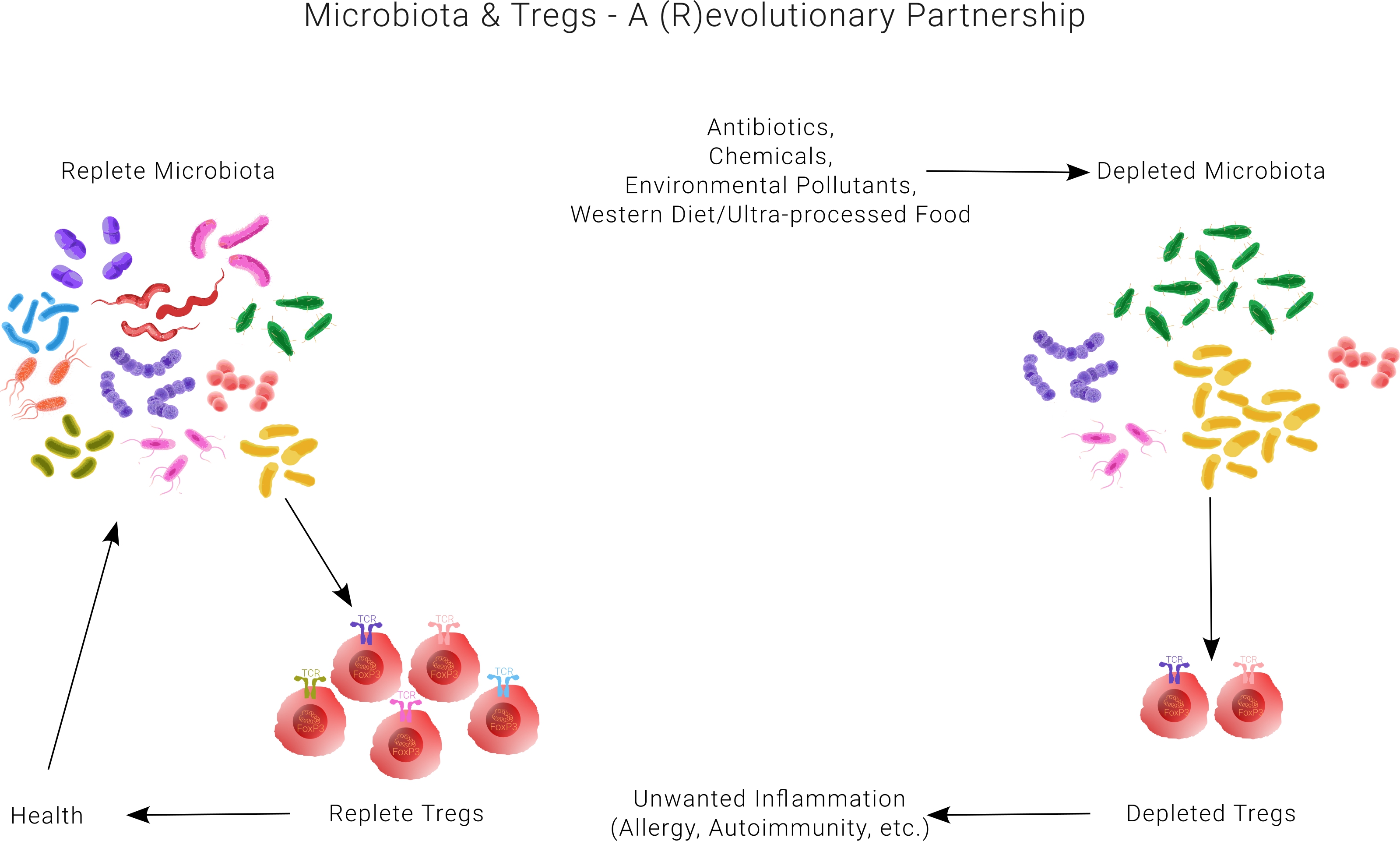
(a) A major challenge in microbiome research is to identify beneficial microbiota species that could support antigen-specific Tregs.
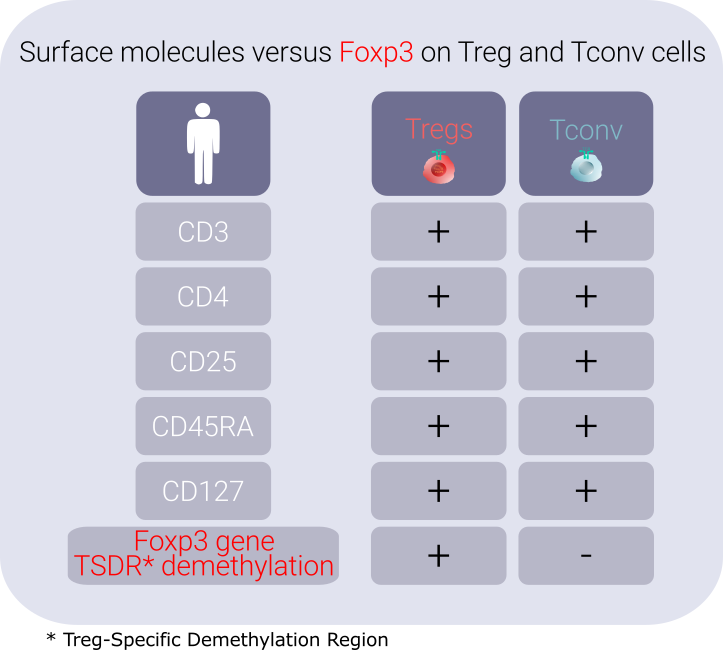
(b) Antigen-specific T cells, called T cell clones, are rare among the total T cell pool, in the range of ~ 1/10,000 – 1/1,000,000, in an unmanipulated host. Additionally, the strategy for isolating viable Tregs is further complicated due to the lack of surface molecules that could reliably differentiate them from conventional T cells (Tconv). The intracellular transcription factor Foxp3 is the only consensus marker that identifies bona fide Tregs and cannot be accessed from outside without destroying them.
Limitations Of Current Approaches
At present, companies predominantly use two different approaches to overcome these challenges:
• First, they aim to modify disease conditions by using a blend of microbiota species without specific consideration for T cell target antigens (Vedanta Biosciences, Enterome, and Microba Life Sciences).This approach relies on serendipitous discoveries — either from animal models or human samples — that reveal associations between particular microbiota species and Treg populations under varying conditions. However, such an approach lacks a systematic and rational foundation to consistently identify disease-relevant microbiota species.
• Second, they engineer Tregs with defined antigen-specificity by transforming them into CAR-Tregs expressing chimeric antigen receptor (Sangamo Therapeutics, Quell Therapeutics, Sonoma Biotherapeutics, Gentibio, TeraImmune, AZTherapies, and Abata Therapeutics) or they use unmodified polyclonal Tregs (Coya Therapeutics, ActiTrexx, Cellenkos). However, such approaches have several limitations:
(a) They disregard the essential role of microbiota in Treg functionality;
(b) They are limited to a few known target antigens;
(c) The T cell source of such CAR-Tregs is unreliable, as there is a high likelihood that T cells from non-Treg lineages could be transformed into actual CAR-T cells that could worsen disease progression rather than alleviate it.
How does Tregeutix differ?
Our unique approach entirely bypasses these challenges. To begin, we went back to the drawing board and developed a novel Treg-focused framework, which we call SPIRAL (Specific ImmunoRegulatory Algorithm). It delineates the mechanisms by which select microbiota species could support antigen-specific Tregs and explains how disruption of such relationships is the root cause of autoimmunity and allergies.
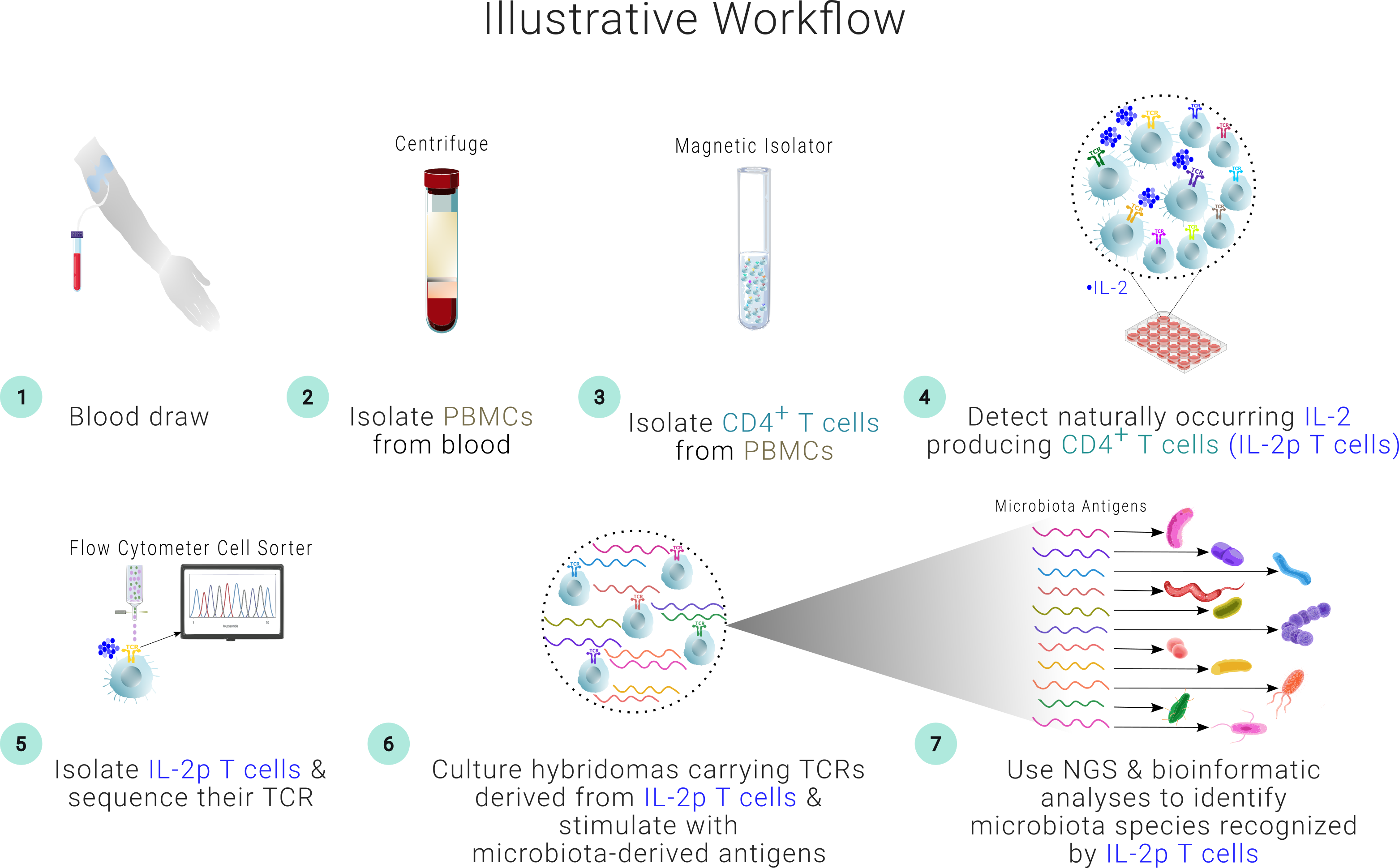
SPIRAL provides a first-in-kind roadmap to identify those select microbiota species that support antigen-specific Tregs. IL-2 is a cytokine essential for the proper function of Tregs. SPIRAL predicts that naturally-occurring IL-2 producing T cells are mirror images of Foxp3+ Tregs with shared antigen-specificity. Thus, screening for this specialized subset of IL-2 producing T cells when exposed to microbiota antigens in an in vitro assay could allow probing for Treg antigen specificity indirectly without the need to manipulate Tregs themselves.
#This figure contains elements adapted from images by the DataBase Center for Life Science, as well as by studiogstock and brgfx on Freepik.
The development of this in vitro screening assay would circumvent challenges associated with Treg-based cell therapies and allow precise identification of those microbiota species that are responsible for maintaining the Treg pool. We will then utilize our proprietary databank of such microbiota species for both prognostic and therapeutic purposes in autoimmune diseases and allergies.
The SPIRAL discovery platform would form the basis for the development of our immunotherapeutic pipeline. The company intends to license its assets to large pharmaceutical companies in return for upfront, milestone and royalty payments.
Founders and Management Team
The company’s founders have extensive knowledge and expertise in developing technically challenging cellular and microbial in vitro assays. They have contributed crucial data in support of several patent applications (Patent No.: US 7,220,718 B2 (Oral treatment of hemophilia); Patent No.: US 7,867,974 B2 (Induction of tolerance by oral administration of factor VIII); Patent No.: US 11,156,601 B2 (In vitro neonatal biomimetic (nMIMIC) model). Their previous experience encompasses academic roles as postdoctoral researchers at the National Institutes of Health (NIH), one of the world’s leading biomedical research centers, as well as positions in the biopharmaceutical industry at Sanofi Pasteur, one of the largest global vaccine developers. They are co-authors of the innovative framework known as SPIRAL, which lays the scientific groundwork for the company’s business plan. SPIRAL introduces a fundamentally new approach to understanding the adaptive immune system. Under development since 2016, various aspects of SPIRAL have been published in peer-reviewed journals across three installments from 2018 to 2022.
Funding Request and Investment Opportunity
The company is currently seeking to raise up to $1,235,000 seed funding to conduct the first proof of concept experiments to validate our idea and to demonstrate the feasibility of our approach. The company plans to use the results of these experiments to attract more financing from established venture firms or institutional funds, and advance product development.
On behalf of the Board of Directors of Tregeutix, we invite you to become an investor in the company. By investing in Tregeutix, you will not only support an original approach for treating autoimmune diseases and allergies with microbiota guided immunotherapies, but also gain early access to an investment opportunity in a biotech company at an early stage when it is traditionally restricted to a small group of wealthy individuals, and when the potential returns are higher and the entry barriers are lower. This is a rare opportunity to contribute to a future where autoimmune diseases and allergies could be safely and effectively managed with the help of rationally selected beneficial microbiota species that modulate the immune system.
However, as with any investment, there are alsorisks involved. Tregeutix is a new startup that has not yet proven its technology or business model. There is no guarantee that the company will be able to achieve its milestones, secure further funding, or obtain regulatory approval. The market for microbiome therapeutics is also highly competitive and dynamic, and the field of microbiome research is still evolving. There may be unforeseen challenges or limitations that could affect the development and commercialization of the company's products.
Therefore, before making any investment decision, you should carefully evaluate the information provided by Tregeutix and consult with your own financial, legal, and tax advisors. You should also be prepared to lose all or part of your investment, as there is no guarantee of success or profitability.
If you are interested in learning more about Tregeutix and its novel platform for identifying and manipulating specific microbiota species that regulate the immune system, you can contact us for more details.
Thank you for your attention and interest in Tregeutix.
Sincerely,
Tregeutix Inc.
We are planning to launch our offerings under Securities Act Section 4(a)(6) and Regulation Crowdfunding (Regulation CF, § 227.100 et seq.), and we want to hear from you! If you are an accredited investor or a non-accredited investor who meets the investment limits under Regulation CF (read here), let us know how much you would be interested to invest in our offering. Your response is not binding and does not obligate you to invest in our offering.
The “test-the-waters” provision permits companies to make informal offers to investors to gauge market interest before starting the Regulation CF process and filing with the Securities and Exchange Commission (SEC). If you are interested in learning more about this potential offering, contact the company via the email listed below and provide your name, address, telephone number, and/or email address, so you will be notified directly to invest once the offering is conducted.